

What is PROST?
PROST is a multicentre, randomized, open-label, blinded outcome, adaptive, non-inferiority trial comparing the pRESET stent-retriever with the Solitaire stent-retriever for large vessel occlusion stroke treatment.
Key Outcomes
ITT pRESET54,91%
90d mRS ≤ 2 84,93%
eTICI ≥ 2b50 ≤ 3 Passes 43,68%
eTICI ≥ 2c 1st PassKey Takeaways
PROST is the first randomized clinical trial aiming to compare a novel versus an established stent-retriever technology, establishing a new scientific benchmark for stroke device trials.
Thrombectomy with the pRESET stent-retriever was found to be non-inferior to thrombectomy with the Solitaire stent-retriever in terms of flow restoration and disability reduction in patients with large vessel occlusion stroke (LVOS).
The PROST Trial led to the FDA clearance of the pRESET device on January 20th 2023 with labels for improvements in both revascularization and clinical outcomes.
Discover pRESET Features

Helical Slit
- Maintain cell shape integrity independent of expansion diameter
- Improved clot retention

Multi Cell Size
- Deep clot integration & improved flexibility in tortuous anatomies
- Improved clot retention & trackability
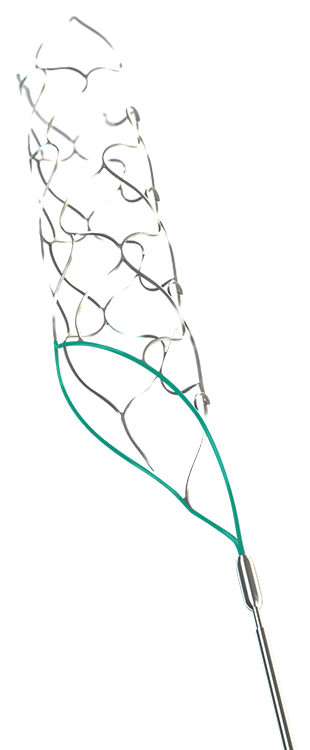
Closed Proximal Ring
- Ensures stable opening and constant wall apposition during retrieval
- Improved clot retention & recapture

